Background
Cancer is a major health problem and a cause of death worldwide. Globally, the number of cancer-related deaths increased significantly to 18.1 million in 2020 (World Cancer Research Fund International, 2022), and it is expected to rise to 27.5 million in 2040 (Cancer Research UK, 2022). In Thailand, cancer was the leading cause of death, with an increasing mortality rate per 100,000 people from 119.30 in 2016 to 129.50 in 2020 (Thai Ministry of Public Health, 2020). Advanced cancer refers to cancer metastasis from its original site or recurrence (Cancer Council NSW, 2018) and can be unresponsive to conventional treatment methods. The number of new Thai patients with advanced cancer rose from 31.40% in 2019 (Thai National Cancer Institute, 2020) to 37.20% in 2021 (Thai National Cancer Institute, 2021). Advanced cancer is often managed with a combination of treatments, depending on the type and location of cancer (Miller et al., 2022). Therefore, patients with advanced cancer experience suffering from both the cancer itself and a combination of cancer treatments.
Patients with advanced cancer encounter many impacts. The physical impacts usually include pain, nausea, shortness of breath, fatigue, constipation, appetite loss, and sleep problems (Bittencourt et al., 2021). If not properly managed, these symptoms lead to profound psychosocial and spiritual impacts, including psychosocial distress, especially depression and anxiety (Bittencourt et al., 2021), and lower quality of life (Kolsteren et al., 2022). In addition, patients may develop a persistent feeling of uncertainty about diagnosis and prognosis and fears of progression, treatment effects, and dying (Kolsteren et al., 2022). Moreover, the expected survival time for advanced cancer ranged from 9 to 12 months (Nahm et al., 2022). Thus, patients need the ability to adapt successfully during the disease process and treatment course, known as resilience.
Resilience is a person’s ability to achieve successful adaptation outcomes (Kumpfer, 1999). Resilience could influence the way patients deal with advanced cancer and treatments through a cognitive reevaluation of the circumstance and the identification of meaning, which finally facilitates adaptation to cancer and improved general well-being (Palacio & Arias, 2018). Unfortunately, patients with advanced cancer had only moderate levels of resilience (Kavak et al., 2021) due to concerns about death and suffering from side effects of treatments that resulted in physical and psychological pain (Tamura, 2021). Moreover, compared to other cancer stages, patients with advanced cancer reported the lowest level of resilience (Aman & Akhtar, 2020). This indicates the need to improve resilience and understand the factors influencing resilience in this population.
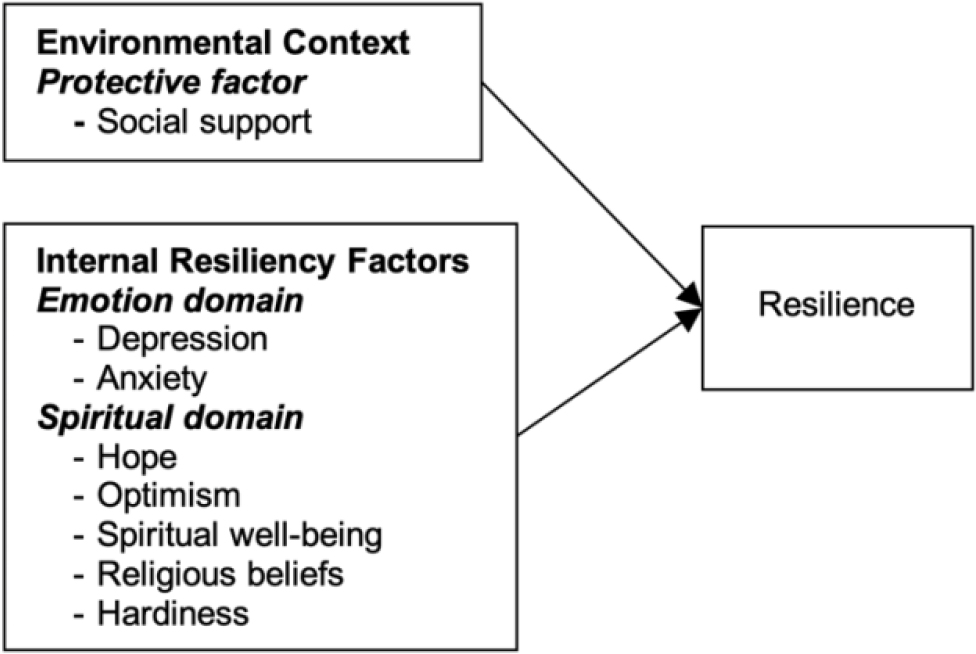
The Resilience Framework of Kumpfer (1999) has been used to explain the complex transactional resilience process in the cancer population (Li et al., 2019). Based on this framework, the resilience process is triggered by stressors or challenges from a person’s surroundings. There are two processes of resilience. Firstly, the person-environment transactional process is an interaction when stressors are transacted to the person. This process is influenced by two factors, including risk and protective factors, which inhibit or promote the impact of the stressor. The strategies used to adapt and modify the environment in this process include perception, reframing, changing environment, and active coping. Secondly, the resiliency process is the interaction between five internal resiliency factors (cognitive, emotional, physical, behavioral, and spiritual domains) and resilience outcomes. This process is used to maintain homeostasis and adaptation. In this study, based on Kumpfer’s model, resilience is influenced by both the environmental context and internal resiliency factors. In the environmental context of patients with advanced cancer, social support is conceptualized as a protective factor that decreases the severity of stress from advanced cancer. For internal resiliency factors, depression and anxiety are conceptualized from the emotional domain representing the feelings of patients with advanced cancer. Hope, optimism, spiritual well-being, religious belief, and hardiness are conceptualized from the spiritual domain (Figure 1).
Social support refers to patients’ perceived aids and supports, including emotional, tangible, and informational supports (Schaefer et al., 1981). It facilitates coping strategies and promotes psychological adaptation to adversity by buffering against stress (Kumpfer, 1999). Previous studies reported significant positive correlations of social support with resilience in oral cancer (Gao et al., 2019), lung cancer (Hu et al., 2018), older adults cancer (Limwattanathawornkul et al., 2019), and prostate cancer (Zhao et al., 2021). In addition, social support predicted resilience in liver cancer (Li et al., 2019) and prostate cancer (Zhao et al., 2021) patients. However, most earlier research investigated correlations between social support and resilience, but there was limited research on the predictive ability of social support on resilience, specifically on advanced cancer.
Depression refers to patients’ mood disorder characterized by sadness and inability to enjoy or show interest (World Health Organization, 2017). Depressed persons are less able to maintain or restore their psychological adaptation when faced with stressful life events (Kumpfer, 1999). Depression was negatively correlated with resilience in lung cancer (Hu et al., 2018), mixed cancer (Mungase et al., 2021), and colorectal cancer (Tamura, 2021). Previous research examined the relationships, but investigations are scarce on the predicting ability of depression on resilience among adult patients with advanced cancer.
Anxiety refers to patients’ feelings of fear or discomfort, including nervousness or worry about something occurring or may occur in the future (World Health Organization, 2017). Persons with anxiety may not believe in their proactive ability to bounce back from adversity (Kumpfer, 1999), as they are uncertain and cannot concentrate on their future goals. If anxiety is reduced, patients with advanced cancer may be able to focus on their problems and adapt to their adverse situations successfully. Anxiety was negatively related to resilience among lung cancer patients (Hu et al., 2018) and colorectal cancer (Tamura, 2021). Despite correlational studies in cancer patients, there was no research on the predictive ability of anxiety on resilience, particularly among patients with advanced cancer.
Hope refers to patients’ dynamic inner capacity that facilitates a new positive perspective of living (Herth, 1992). Hope may motivate emotional stability to maintain focus and success in life (Kumpfer, 1999). Hopeful patients can deal effectively with cancer-related hardship, providing adaptive power to overcome difficult situations and perceiving obstacles as challenges to overcome (Costa et al., 2019). Thus, hopeful patients with advanced cancer may develop a new positive way to view their situation and adapt to their advanced cancer experience. Reports showed that hope was positively correlated with resilience among oral cancer (Gao et al., 2019) and older people cancer (Limwattanathawornkul et al., 2019) patients. Moreover, hope positively predicted resilience among patients with liver cancer (Li et al., 2019) and prostate cancer (Zhao et al., 2021). Despite investigations on correlations and predictive ability between hope and resilience in the cancer population, previous research did not focus on adult patients with advanced cancer.
Optimism refers to patients’ enduring personality traits associated with positive expectations about future events (Scheier et al., 1994). As people anticipate positive future events, they can bounce back after adversity (Kumpfer, 1999). In the context of advanced cancer, high optimism may increase patients’ ability to successfully adjust to cancer, accept the challenges caused by cancer, and use more adaptive strategies to cope (Gallagher et al., 2019). In addition, optimism was positively correlated with resilience in oral cancer patients (Gao et al., 2019). Previous research focused on correlations between optimism and resilience in cancer, but no research with regression analysis was found, particularly among patients with advanced cancer.
Spiritual well-being refers to the self-perception of having a peaceful and happy life, understanding of self and the nature of life, and feeling connected and hopeful (Promkaewngam et al., 2014). It helps people understand life’s purpose and motivates them to overcome adversity (Kumpfer, 1999). Thus, higher spiritual well-being may increase patients’ ability to adapt to adversity successfully. Spiritual well-being had a positive correlation with resilience in advanced gastrointestinal cancer (Kavak et al., 2021). Spiritual well-being was positively associated with resilience among 636 patients with advanced cancer (Mihic-Gongora et al., 2022). Unfortunately, it was studied only for correlation with resilience in patients with advanced cancer in a Western country.
Religious belief refers to the mental representation of patients’ attitudes positively oriented toward Buddha and the Buddhist doctrine as being true, particularly one without a proof (Seesopon et al., 2017). Religious belief is a powerful inner source crucial for a person’s successful adaptation (Kumpfer, 1999). Patients with strong religious beliefs may find relief and draw strength to continue coping with their disease and adapt to adverse situations caused by cancer. Religious belief was positively correlated with resilience among cancer patients (Al Eid et al., 2020) and predicted resilience among patients with breast cancer (Fradelos et al., 2018). Although prior works examined the correlation and predictive ability of religious belief on resilience in cancer patients, there was limited research on Buddhist religious belief and the predictive ability, particularly in patients with advanced cancer.
Hardiness refers to patients’ personality traits for adapting to the present or possible health issues through control, challenge, and commitment (Pollock & Duffy, 1990). It is a powerful inner source necessary for people to adapt to challenging situations successfully (Kumpfer, 1999). Hardiness is theoretically essential to resilience as it makes individuals confident in appropriately appraising and modifying their actions, thereby increasing motivation and competence to deal with difficulty (Pollock & Duffy, 1990). In addition, patients with hardiness may be confident in controlling symptoms from cancer treatments and cancer itself and view advanced cancer as an opportunity for psychological growth (Seiler & Jenewein, 2019). Therefore, higher hardiness may bring a greater ability to adapt successfully to cancer-related distress. Unfortunately, nowadays, research to examine hardiness in adult patients with advanced cancer is lacking.
From the evidence above, depression, anxiety, optimism, and spiritual well-being were studied mostly for correlations to resilience in adult cancer patients, some of whom had advanced cancer. In addition, social support, hope, and religious belief were examined on resilience in cancer patients. Hardiness remains uninvestigated. Thus, there is a little investigation on the relationship and the predicting ability of those factors with resilience in adult patients with advanced cancer. In Thailand, patients with advanced cancer receive their treatments only at tertiary care hospitals and university hospitals (Thai Ministry of Public Health, 2023).
Moreover, 92.52% of the Thai population are Buddhist (Department of Religious Affairs, 2020) and may hold different views toward life with cancer. With such a distinctive scenario, the characteristics of patients with advanced cancer in Thailand may differ significantly from other settings, and research from Western perspectives may not be applicable to Thai patients. Therefore, this research aimed to examine the influence of social support, depression, anxiety, hope, optimism, spiritual well-being, religious belief, and hardiness on resilience among Thai adult patients with advanced cancer. The multiple regression formula of this study was Y (resilience) = β0 + β1 (social support) + β2 (depression) + β3 (anxiety) + β4 (hope) + β5 (optimism) + β6 (spiritual well-being) + β7 (religious belief) + β8 (hardiness) + €. The hypothesized model is illustrated in Figure 1.
Methods
Study Design
A cross-sectional design was used.
Samples/Participants
The sample size was calculated for multiple regression analysis with a ratio of subjects to independent variables 30:1 (Hair et al., 2014). With eight independent variables, the sample size was 240 plus a 20% attrition rate (Gray et al., 2016). Therefore, the samples of this study were 288.
The participants were selected using multi-stage sampling. First, all university hospitals and tertiary hospitals in northern Thailand were identified. Second, four hospitals were selected based on a proportion of all university and tertiary hospitals in the region using simple random sampling by lottery technique without replacement, resulting in one university hospital and three tertiary hospitals. Third, eligible patients with advanced cancer were selected from each hospital using purposive sampling based on the proportion. The inclusion criteria were: 1) adult aged 30-60 years, newly diagnosed for at least one month by a physician with any type of advanced cancer (metastatic [M1], Stage IV, or distant cancer for solid cancer; Stage III or IV for hematologic cancer); 2) a moderate level of symptoms, with at least two symptoms, indicated by a score of 4-6 assessed using Visual Analog Scale from Edmonton Symptom Assessment System (ESAS); 3) able to perform self-care, assessed with Palliative Performance Scale (PPS) with a score between 80 and 100; 4) cognitively intact assessed with Montreal Cognitive Assessment Thai version, with a score of at least 25; 5) Buddhist; 6) able to read, write, communicate, and understand the Thai language; and 7) participation with willingness. Patients with advanced cancer were excluded if they had breathing difficulty and pain indicated by a score of 7-10 on the ESAS and had cancer spreading to the brain as diagnosed by a physician.
Instruments
Nine instruments were used. All instruments, except for the demographic data collection form, were used with permission from the original authors. In addition, the Herth Hope Index (HHI), Life Orientation Test-Revised (LOT-R), and Health-Related Hardiness Scale (HRHS) were translated into Thai by the researcher using back-translation of Brislin (1970) with permission from the original authors. The content validity of the translated instruments was reviewed by six experts, including two oncologists, two oncology nurses, and two nursing instructors. Each instrument is described as follows:
The demographic data form created by the researcher included age, gender, education, income, advanced cancer duration, cancer type, type of treatment during hospital admission, and symptoms during hospital admission.
The Thai version of the Social Support Questionnaire (SSQ), created by Schaefer et al. (1981) and translated into Thai and modified by Hanucharurnkul (1988), was adopted to assess social support. It had two parts. Part 1: sources of social support from 1) family, 2) cancer patients, 3) health care professionals, 4) friends and neighbors, and 5) supervisors or co-workers. Patients selected one or more sources. Part 2: social support types comprised seven items in 3 subscales, including emotional support (4 items), tangible (2 items), and informational support (1 item). Items were rated on a 5-point Likert scale from 0 (perceived no support) to 4 (perceived the most support). All Part 2 items were summed for a total score. The total possible score ranged from 0 to 28, and higher scores reflected more social support (Schaefer et al., 1981). Cronbach’s alphas were 0.71 (n = 10) and 0.81 (n = 288).
The Hospital Anxiety and Depression Scale (HADS) was used to measure anxiety and depression. It was created by Zigmond and Snaith (1983) and translated into Thai by Nilchaikovit et al. (1996). It comprised 14 items in anxiety (7 items) and depression (7 items). Items were rated on a 4-point Likert scale from 0 (absence of symptoms) to 3 (frequent presence of symptoms). Scoring was performed separately for anxiety and depression subscales. All items in each subscale were summated to obtain a total score. The total score ranged from 0 to 21 for each subscale, where scores of 0-7 represented normal cases; 8-10 for borderline abnormal cases; and 11-21 represented abnormal cases (Zigmond & Snaith, 1983). Cronbach’s alphas were 0.84 (n = 10) and 0.85 (n = 288) for anxiety and 0.68 (n = 10) and 0.93 (n = 288) for depression.
The Herth Hope Index (HHI) was utilized to assess hope. It was created by Herth (1992). It had 12 items with two negative items (items 3 and 6). Items were rated on a 4-point Likert scale between 1 (strongly disagree) and 4 (strongly agree). Negatively worded items were reverse-scored. Total possible scores ranged from 12 to 48, where higher scores represented greater hope (Herth, 1992). The content validity index for scale (S–CVI) was 1. Cronbach’s alphas were 0.83 (n = 10) and 0.73 (n = 288).
Life Orientation Test-Revised (LOT-R) was adopted to measure optimism. It was created by Scheier et al. (1994). It had ten items about life orientation: optimism (3 items), pessimism (3 items), and filler items (4 items), rated on a 5-point Likert scale from 0 (strongly disagree) to 4 (strongly agree). All pessimism items were negatively worded and reverse scored. Total possible scores ranged between 0 and 40, where higher scores reflected greater optimism (Scheier et al., 1994). In this study, S–CVI was 0.95. Cronbach’s alphas were 0.74 (n = 10) and 0.70 (n = 288).
Spiritual Well-being Scale for Thai Buddhist Adults with Chronic Illness (SWS-TBACI) created by Promkaewngam et al. (2014) was used to assess spiritual well-being. It contained 13 items: 1) being happy (4 items); 2) understanding of self and nature of life (4 items); and 3) hope and sense of connectedness (5 items) rated on a 5-point Likert scale between 1 (strongly disagree) and 5 (strongly agree). All items were calculated to yield a total mean score. The total mean score ranged from 1 to 5 and was divided into three ranges: 1.00-2.33 (low); 2.34-3.67 (moderate); and 3.68-5.00 (high) spiritual well-being (Promkaewngam et al., 2014). Cronbach’s alphas were 0.90 (n = 10) and 0.93 (n = 288).
The Buddhist Belief Questionnaire developed by Seesopon et al. (2017) was employed to measure Buddhist religious belief. It contained 18 items in two components: belief in Four Faiths (14 items) and belief in Three Marks of Existence (4 items). Items were rated on a 5-point Likert scale between 1 (slightly agree) and 5 (strongly agree). The total possible score ranged between 18 and 90, with higher scores meaning stronger Buddhist religious belief and divided into three ranges: low (18-42), moderate (43-66), and high (67-90) (Seesopon et al., 2017). Cronbach’s alphas were 0.77 (n = 10) and 0.96 (n = 288).
Health-Related Hardiness Scale (HRHS) was employed to assess hardiness. It was created by Pollock and Duffy (1990). It contained 34 items: 14 for control, seven for commitment, and 13 for challenge. Items were rated on a 6-point Likert scale between 1 (strongly disagree) and 6 (strongly agree), with 23 negatively worded items that were reverse scored. The total possible score ranged from 34 to 204; higher scores meant greater health-related hardiness (Pollock & Duffy, 1990). In this study, S–CVI was 0.97. Cronbach’s alphas were 0.82 (n = 10) and 0.69 (n = 288).
The Connor-Davidson Resilience Scale (CD-RISC) was adopted to assess resilience. It was created by Connor and Davidson (2003) and is available in a Thai version from the original authors (J. Davidson, personal communication, July 19, 2019). It comprised 25 items in five domains: 1) 8-item personal competence, 2) 7-item tolerance of negative effect, 3) 5-item positive acceptance of change, 4) 3-item control, and 5) 2-item spirituality. Items were rated on a 5-point Likert scale between 0 (not true at all) and 4 (true nearly all the time). Total possible scores ranged between 0 and 100, where a higher score indicated higher resilience (Connor & Davidson, 2003). Cronbach’s alphas were 0.77 (n = 10) and 0.93 (n = 288).
Data Collection
Data were collected from February 2021 to February 2022. Three research assistants were recruited based on the criteria: 1) being nurses working at in-patient medical or surgical wards and 2) having a minimum of two years of experience in advanced cancer care. They were trained by the researcher for participant recruitment, informed consent, and data collection procedure. Participants were selected from the medical records of all in-patients with advanced cancer in each hospital. They were screened for eligibility based on the inclusion criteria. After agreeing to participate and signing an informed consent, the researcher or assistants read the questionnaires, and the participants responded. The total time to complete was 60 to 90 minutes with a 15-minute break.
Data Analysis
Data analysis was run with SPSS version 13.0. All data were screened for accuracy and completeness before data analysis. There were no missing data. The analysis of demographic variables was run with descriptive statistics. The relationships between the independent and dependent variables were investigated using Pearson’s and Spearman’s correlation. The correlational model was analyzed using the multiple regression analysis with enter method. All assumptions for multiple regression analysis were met.
Ethical Consideration
Approval was granted by the Research Ethics Committee of the Faculty of Nursing, Chiang Mai University (Research ID: 2562-132; Study Code: 2562-FULL044). The researchers or the research assistants explained to participants about the study information, including objectives, methods, and time required to complete the questionnaires. Participants were free to decide to participate and to refuse or discontinue participation at any time until data collection was concluded without negative effects on their treatment. Following the agreement to participate, a consent form was signed. They were thanked and given a token. In addition, a code number was assigned to assure anonymity and confidentiality.
Results
Characteristics of the Participants
From Table 1, all 288 participants were Buddhist, aged 30 to 60 (mean = 51.35, SD = 7.52). They were male (52.40%) and female (47.60%), graduated from primary school (37.80%), and had the highest income of 303.15-909.37 USD/month (44.70%). The mean duration of having advanced cancer was 4.12 months (SD = 2.16).
| Demographic Characteristics | Frequency | % |
|---|---|---|
| Age (years) Range 30-60 years (mean = 51.35 years, SD = 7.52 years) | ||
| 30-40 | 30 | 10.40 |
| 41-50 | 75 | 26.10 |
| 51-60 | 183 | 63.50 |
| Gender | ||
| Male | 151 | 52.40 |
| Female | 137 | 47.60 |
| Education | ||
| Primary | 109 | 37.80 |
| Secondary | 79 | 27.40 |
| Diploma | 44 | 15.30 |
| Bachelor degree or higher | 56 | 19.50 |
| Income (USD per month) | ||
| < 303.12 | 115 | 39.70 |
| 303.15-909.37 | 130 | 44.70 |
| 909.40-1,818.73 | 37 | 12.50 |
| > 1,818.76 | 6 | 3.10 |
| Duration of advanced cancer (months) (Mean = 4.12, SD = 2.16) | ||
| 1-5 | 193 | 67.00 |
| 6-10 | 95 | 33.00 |
| Type of cancer | ||
| Colorectal | 73 | 25.30 |
| Liver | 42 | 14.60 |
| Breast | 31 | 10.80 |
| Lung | 30 | 10.40 |
| Lymphoma | 25 | 8.70 |
| Ovary | 18 | 6.20 |
| Cervix | 12 | 4.20 |
| Leukemia | 8 | 2.80 |
| Other (Solid in a variety of organs/systems) | 49 | 17.00 |
| Treatment during hospital admissiona | ||
| Chemotherapy | 288 | 100.00 |
| Surgery | 41 | 14.20 |
| Radiation | 26 | 9.00 |
| Hormone therapy | 7 | 2.40 |
| Other (symptomatic treatment) | 19 | 6.60 |
| Symptoms during hospital admissiona | ||
| Fatigue | 167 | 58.00 |
| Nausea | 149 | 51.70 |
| Pain | 137 | 47.60 |
| Weight loss | 79 | 27.40 |
| Vomiting | 69 | 24.00 |
| Constipation | 64 | 22.20 |
| Tiredness | 46 | 16.00 |
| Other | 117 | 40.60 |
The three most frequently reported types of cancer included colorectal cancer (25.30%), liver cancer (14.60%), and breast cancer (10.80%). The participants received combined treatments, including chemotherapy (100%), surgery (14.20%), and radiation (9.00%). The most frequently reported symptoms during hospital admission were fatigue (58.00%), nausea (51.70%), and pain (47.60%).
Range, Mean, and Standard Deviation of Study Variables
Table 2 shows that the mean score of social support was 23.52 (SD = 3.47). The mean score for depression and anxiety was 5.38 (SD = 4.90) and 5.19 (SD = 4.48), respectively, indicating that most participants were normal cases. The mean score of hope, optimism, spiritual well-being, religious belief, hardiness, and resilience was 35.89 (SD = 4.25), 16.07 (SD = 2.81), 53.24 (SD = 7.78), 73.78 (SD = 11.50), 130.48 (SD = 11.14), and 76.11 (SD = 12.90) respectively.
| Variables | Possible Score | Actual Score | Mean | SD |
|---|---|---|---|---|
| Social support | 0-28 | 11-28 | 23.52 | 3.47 |
| Depression | 0-21 | 0-18a | 5.38 | 4.91 |
| Anxiety | 0-21 | 0-15b | 5.11 | 4.17 |
| Hope | 12-48 | 24-45 | 35.89 | 4.25 |
| Optimism | 0-40 | 6-24 | 16.07 | 2.81 |
| Spiritual well-being | 13-65 | 25-65 | 53.24 | 7.78 |
| Religious belief | 18-90 | 36-90 | 73.78 | 11.50 |
| Hardiness | 34-204 | 95-175 | 130.48 | 11.14 |
| Resilience | 0-100 | 25-100 | 76.11 | 12.90 |
Note: a = 0-7 n = 205 (71.20%), 8-10 n = 30 (10.40%), 11 n = 53 (18.40%)
b = 0-7 n = 222 (77.10%), 8-10 n = 28 (9.70%), 11 n = 38 (13.20%)
Relationships between Independent Variables and Resilience
Statistical assumptions for normality were tested using Kolmogorov-Smirnov. The data that met the assumptions were analyzed with Pearson’s correlation, while Spearman rank correlation was used for data that violated the assumptions. From Table 3, spiritual well-being had a highly positive relationship with resilience. Hope and religious belief had a moderate positive relationship with resilience. Anxiety and depression had low negative relationships with resilience, whereas social support and optimism had low positive relationships with resilience. Finally, hardiness had a very low positive relationship with resilience.
| Independent Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Social supportb | 1 | ||||||||
| 2. Depressionb | 0.34** | 1 | |||||||
| 3. Anxietyb | 0.34** | 0.84** | 1 | ||||||
| 4. Hopeb | 0.32** | -0.38* | -0.31** | 1 | |||||
| 5. Optimismb | 0.12* | -0.09 | -0.02 | 0.30** | 1 | ||||
| 6. Spiritual well-beingb | 0.44** | 0.58** | -0.45** | 0.25** | 0.49** | 1 | |||
| 7. Religious beliefb | 0.34** | 0.35** | -0.28** | 0.25** | 0.37** | 0.54** | 1 | ||
| 8. Hardinessa | 0.03 | 0.11 | 0.16 | 0.20** | 0.30** | 0.25** | 0.25** | 1 | |
| 9. Resilience | 0.33** | -0.47** | -0.39** | 0.67** | 0.40** | 0.74** | 0.53** | 0.21** | 1 |
Factors Influencing Resilience
Seen from Table 4, spiritual well-being and hope could influence resilience at a statistical significance of p <0.01, with spiritual well-being as the strongest influencing factor. However, social support, depression, anxiety, optimism, religious belief, and hardiness could not significantly affect resilience.
| Influencing factors | B | SE | β | t | p-value |
|---|---|---|---|---|---|
| (Constant) | -87.951 | 15.217 | -5.780 | 0.000 | |
| Spiritual well-being | 0.976 | 0.075 | 0.589 | 12.981 | 0.000* |
| Hope | 72.228 | 11.230 | 0.292 | 6.432 | 0.000* |
| Social support | 0.060 | 1.526 | 0.128 | ||
| Depression | -0.050 | -1.152 | 0.250 | ||
| Anxiety | -0.041 | -1.040 | 0.299 | ||
| Optimism | 0.031 | 0.703 | 0.483 | ||
| Religious belief | 0.041 | 0.877 | 0.381 | ||
| Hardiness | 0.007 | 0.181 | 0.856 |
Note: R = 0.806, R2 = 0.649, Adjusted R2 = 0.647, R2 Change = 0.051, F (1,285) = 41.364*, p <0.01
From Table 5, spiritual well-being in the first model could influence resilience, accounting for 59.70% of the variance in resilience. In the second model, spiritual well-being and hope could jointly explain 64.70% of the variance in resilience.
Discussion
Our finding showed that both spiritual well-being and hope influenced resilience, which adds new knowledge to the context of patients with advanced cancer in Thailand. These factors were cognitive capabilities or belief systems that motivate patients to create a direction for their efforts to bounce back from negative cancer experiences.
Spiritual well-being could influence resilience. The explanation could be based on a conceptual framework that patients with high spiritual well-being might see their life’s meaning and purpose, which motivated them to adapt to advanced cancer more effectively. They might understand the nature of life and achieve psychological growth from illness. Another explanation is that spiritual well-being had a strong statistical relationship with resilience. Moreover, most participants were adults aged 51-60 years. Age was significantly positively related to spiritual well-being among cancer survivors (Suara et al., 2017), suggesting that the older patients, the better their spiritual well-being. Additionally, most participants were indicated as normal cases of anxiety and depression. An absence of depression and anxiety resulted in higher spiritual well-being (Chen et al., 2021). Consistent with previous research, spiritual well-being predicted resilience among patients with cancer (Mihic-Gongora et al., 2022).
Hope could influence resilience. Based on the resilience model, hope motivates people to continue living, wish for a brighter future, and develop resilience from adversity (Kumpfer, 1999). In addition, hope provides internal strength for fighting against cancer (Li et al., 2019) by acting as a mechanism for adjusting to experience and relieving distress from cancer. Thus, the more hopeful patients are, the more spiritual strength they have to adapt to life with cancer. Cancer treatments also allowed patients to be hopeful that treatments might help prolong their lives and increase their quality of life (Kolsteren et al., 2022). Moreover, all participants in this study were Buddhist. Buddhist teachings propose that illness and death are natural and inevitable to all humans (Somaratne, 2018). Such Buddhist perspectives may help patients accept their condition and improve their resilience. Our finding was consistent with previous studies that hope positively predicted resilience among patients having liver cancer (Li et al., 2019) and oral cancer (Gao et al., 2019).
Social support was not an influencing factor of resilience among patients with advanced cancer. One possible explanation is that social support involves assistance and encouragement for patients to deal with illness (Schaefer et al., 1981). However, resilience is the inner strength to develop a positive mindset shaped by innate values and enduring modifications from the environment (Kumpfer, 1999; Luo et al., 2020). Therefore, although patients perceived high social support from external sources, the social support received might not suit their needs. Moreover, social support had a low positive relationship with resilience in this study, so it might not be strong enough to influence resilience. In contrast, previous research showed that social support significantly predicted resilience in head and neck cancer survivors in Pakistan (Zahid et al., 2019) and in persons with prostate cancer (none of them had advanced cancer) (Zhao et al., 2021). However, in line with our findings, perceived social support could not significantly affect resilience in patients with oral cancer (Gao et al., 2019).
Depression and anxiety could not influence resilience, which contradicted the study framework. A possible explanation is that most participants were normal cases of depression (mean = 5.38, SD = 4.91) and anxiety (mean = 5.19, SD = 4.48). They might not perceive advanced cancer as causing much worry or emotional difficulty that greatly disrupts their equilibrium. Moreover, both depression and anxiety had a moderate negative relationship with resilience, which in turn might not be strong enough to influence resilience. Inconsistently, depression and anxiety were significantly associated with the resilience of cancer survivors (Zahid et al., 2019).
Optimism, religious belief, and hardiness could not influence resilience. This might be due to the low positive relationship of hardiness, and the moderate relationship of optimism and religious belief with resilience, which might not be strong enough to influence resilience. Another possible explanation may be the issues of the related or overlapping concepts and the measurement of the variables in similar concepts. Some variables related to resilience might indirectly affect resilience through spiritual well-being and hope. Resilience, hardiness, and optimism were interrelated concepts (Tedeschi & Calhoun, 2004). Our findings contradicted previous studies that optimism (Gao et al., 2019) and religious belief (Fradelos et al., 2018) could predict resilience in patients with cancer.
Limitations
This study had some limitations. As this research had a cross-sectional design, a temporal relationship between the result and the exposure could not be established. The participants had mixed types of cancer, which might lead to different complaints and affect patients’ resistance.
Implications and Recommendations
The findings highlight the importance of holistic care for patients with advanced cancer. Nurses should provide psychological support to enhance patients’ spiritual well-being and hope to motivate them to live with advanced cancer. In addition, nurses should assess patients’ spiritual needs and tailor interventions to alleviate patients’ suffering that is specific to individual needs. Further research is recommended to develop and test a causal model to examine the relationship between factors with direct and indirect effects on resilience. Moreover, further research should consider specifying the type of cancer and examining more diverse aspects of resilience.
Conclusion
Spiritual well-being and hope were significant factors influencing resilience among adult patients with advanced cancer. The findings confirmed the proposed research hypotheses, which led to the conclusion that the internal resiliency factors in the spiritual domain provide patients with competencies or strengths necessary for the successful adaptation to the cancer experience.